
Start Here
1. Contact the Research Office (research@awh.org.au) - we're here to help you get started!
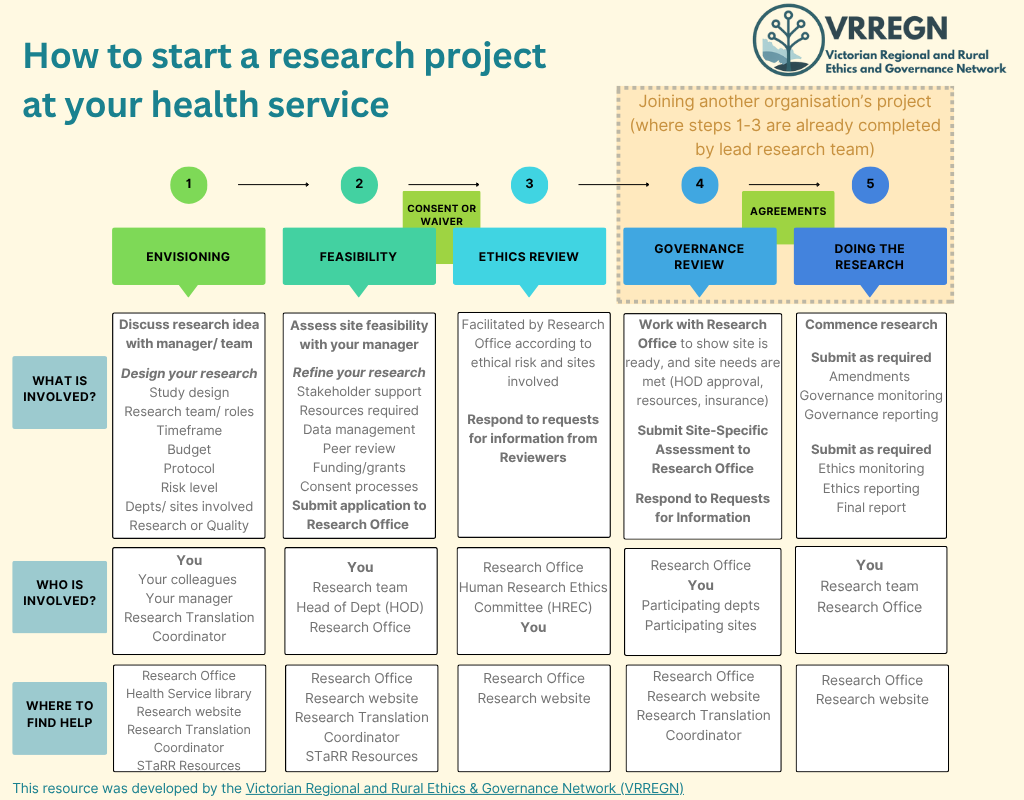
2. Read relevant Research Office Documents (see section below), research guidance material, the fee schedule and the pathway to research flowchart (at the bottom of the page)
3. Complete the GCP training module (and get a certificate) OR find your current GCP certificate
4. Check the AWH Human Research Ethics Committee meeting deadlines for submission dates
5. Create a protocol using the relevant Protocol Template (see section below) and complete the Review Pathway Checklist at the end of the protocol
6. If applicable, create a Participant Information and Consent Form or a Participant Information Sheet using the templates provided below.
7. Submit a Research Request Form for review by the Research Office - attach your draft protocol and PICFs
8. Research request approved? The Research Office will provide guidance on the next steps, such as getting ready for ethical review.
9. Do you want to publish your research? Please let us know! Complete the Request to Publish Form so we can help you get appropriate sign-off. If you're doing a Case Study, please use the AWH Case Study Consent Form to gain consent from your patient.
Doing a Case Study?
If you'd like to do a case study or a case series (≤ 10 people) you don't need approval from the Human Research Ethics Committee. However, you will need institutional approval to publish.
Please use the AWH Case Study Consent Form to gain consent from your patient/s. Attach it to the AWH Request to Publish Form to gain approval to publish. Contact us (research@awh.org.au) if you need help.
Protocol Templates
The Research Office has developed templates to help you form a protocol for your project. Each template includes guidance on each section and is tailored towards project type. Please contact the Research Office (research@awh.org.au) for assistance with your protocol.
Use the Research Protocol Template for general research projects
Use the Clinical Trial Protocol Template if you are initiating a clinical trial
Use the Quality Improvement Project Protocol Template if you are conducting a quality improvement project that may require ethical review (contact the Research Office for guidance)
Participant Information and Consent Form (PICF) templates
The templates below have been tailored for use at AWH. For further guidance, see the Research Office PD and Education page.
PICF for general research and clinical trials
Research Video Series
Hear about the work we do in the Research Office and from some of our clinician researchers.
Project submission due dates
Research Office review
To ensure that research protocols and associated documents are of a high standard before being submitted to the AWHHREC, pre-ethical review of projects will be undertaken by the Research Office. Research Office staff have research experience and are able to assist with protocol development.
Draft protocols for new projects can be submitted to the Research Office 6 weeks before the AWH Human Research Ethics Committee (HREC) meeting date using the Research Request Form. This allows time for the Research Office to review the protocol and associated documents to ensure they are ready for ethical review. After review by the Research Office, researchers will be guided through submission to the AWHHREC on ERM.
Quality Improvement projects will be assessed by the Clinical Safety and Quality Unit and the QI Review Panel. Any Quality Improvement projects that require ethical review will be submitted to the Research Office 6 weeks before the next HREC meeting date.
2026 Project Submission Dates
|
New projects sent to Research Office With draft protocol (6 weeks before meeting) |
New Project submitted in ERM Deadline for new projects (4 weeks before meeting) |
Updated submission due Adjustments to projects due (2 weeks before meeting) |
AWHHREC meeting date You will be invited to attend |
| Wed 18 Feb | Wed 4 Mar | Wed 18 Mar | Wed 1 April |
| Wed 22 Apr | Wed 6 May | Wed 20 May | Wed 3 June |
| Wed 20 May | Wed 3 Jun | Wed 17 Jun | Wed 1 July |
| Wed 24 Jun | Wed 8 Jul | Wed 22 Jul | Wed 5 August |
| Wed 26 Aug | Wed 9 Sept | Wed 23 Sept | Wed 7 October |
| Wed 23 Sept | Wed 7 Oct | Wed 21 Oct | Wed 4 November |
ERM Resources
Researchers need to submit their application through ERM. Refer to the Applicant User Guide to ERM or visit the Clinical Trials and Research website for guidance.
The following training videos may help if you are new to ERM:
🎓 Create a new project and transfer a project
🎓 Create and transfer sub-forms
AWHHREC Governance Documents
Albury Wodonga Health Human Research Ethics Committee Terms of Reference
Members of the Albury Wodonga Health Human Research Ethics Committee (AWHHREC)
The AWHHREC is constituted as per the National Statement on Ethical Conduct in Human Research 2023
|
Name and affiliation |
National Statement member category |
AWH staff? |
|
Annie Hung | Endocrinologist, General Physician | Albury Wodonga Health |
Chairperson |
Yes |
|
Nyra Fell | Practice Manager, Luke Baitch Specialist Anaesthetist |
Community member |
No |
|
Karen Trenchard | Family Room & Education Coordinator | Ronald McDonald House Charities |
Community member |
No |
|
Mark Norden | Medical Director | Women’s & Children’s Health, Albury Wodonga Health |
Professional care of people |
Yes |
|
Bridget Rusike | My Step to Mental Wellbeing Counsellor | Murrumbidgee Local Health District |
Professional care of people |
No |
|
Tapiwa Rusike | Pharmacist | Blooms the Chemist Myrtleford |
Professional care of people |
No |
|
Phillip Steele | Conjoint Senior Lecturer, UNSW Rural Clinical Campus & Recently Retired General Practitioner/Obstetrician |
Professional care of people |
No |
|
Catherine Dawson | Minister of Religion and Pastoral Care Coordinator |Albury Wodonga Health |
Pastoral care |
Yes |
|
Melanie Robb | Retired Lawyer |
Lawyer |
No |
|
Mark Ashcroft | Chief Executive Officer | NCN (Nathalia, Cobram and Numurkah) Health |
Research experience |
No |
|
Kate Freire | Research Fellow | Three Rivers Department of Rural Health, CSU |
Research experience |
No |
|
Tegwyn McManamny | Executive Director, Quality & Clinical Innovation | Ambulance Victoria |
Research experience |
No |
|
Guinever Threlkeld | Head of Campus | LaTrobe University, Wodonga |
Research experience |
No |
|
Hong Yu | Consultant Physician/Geriatrician | Albury Wodonga Health |
Research experience |
Yes |
Library and Research Newsletters
| 2025 | 2024 |
| November 2025 | December 2024 |
| October 2025 | September 2024 |
| August 2025 | July 2024 |
| July 2025 | June 2024 |
| June 2025 | April 2024 |
| May 2025 | March 2024 |
| April 2025 | |
| February 2025 | |
Code of Conduct
Albury Wodonga Health (AWH) is committed to supporting the conduct of safe, quality research to further develop the body of knowledge informing healthcare, particularly as it relates to the community of Albury Wodonga and their healthcare needs. On this page are the key resources, contacts and procedures for undertaking research at AWH to ensure compliance with the Australian Code for Responsible Conduct of Research (2018).
The AWH Research Office follows the core values of:
- honesty and integrity in research;
- respect for human research participants;
- good stewardship of public resources to carry out research;
- appropriate acknowledgment of those involved in research;
- responsible communication of research results.
The Guide to Managing and Investigating Potential Breaches of the Australian Code for the Responsible Conduct of Research (2018) outlines the framework for investigating and managing potential breaches of the Code. This framework is reflected in the AWH Complaints Procedure.
Fee Schedule for Ethics and Governance 💲
The fees for the submission of research applications are available on our website and apply to non-AWH staff. Invoices for governance and ethics fees are sent to the Principal Investigator. Ethics fees are only associated with projects requiring full Human Research Ethics Approval.
Publishing Your Research 📚
Please complete the Request to Publish - AWH Staff or Data form so we can get appropriate sign-off for you.
This includes case studies, conference proceedings and research papers.
Conducting a clinical trial at AWH?
Register your clinical trial
Your trial should be registered with the Australian and New Zealand Clinical Trials Registry. If you haven’t already done this, please do so as soon as possible so that your project is registered before the first participant is recruited. Please pass on the details of the registration to the Research Office so we have a record.
The clinical trial must comply with all relevant Commonwealth laws and regulations, including:
- The Therapeutic Goods Act 1989 (Cth) (TG Act)
- National Health and Medical Research Council Act 1992
- The Therapeutic Goods Regulations 1990 (Cth)
- Privacy Act 1988 (Cth)
- Privacy Act 2000 (Vic)
- Health Records Act 2001 (Vic)
Resources:
- The Australian Clinical Trials website
- Victorian Clinical Trials Education Centre (V-CTEC)
- This includes free training and resources for those conducting clinical trials. You just need to register to gain access.
- NHMRC’s eLearning module: Clinical Trials Environment in Australia
- Information, resources and training videos
- Victorian Coordinating Office for Clinical Trials and Research
- Information on the governance of clinical trials in Victoria
- Support with ERM
- NHMRC guidelines:
- Australian code for the responsible conduct of research
- National Statement on Ethical Conduct in Human Research (2023)
- Risk-based management and monitoring of Clinical Trials involving therapeutic Goods 2018
- Reporting of Serious Breaches of Good Clinical Practice (GDP) or the Protocol for Trials Involving therapeutic Goods
- NHMRC Guidance: Safety monitoring and reporting in clinical trials involving therapeutic goods November 2016
- Guide to Managing and Investigating Potential Breaches of the Code (2018)


.png-639053714440973964.png)


